Increasingly, we have seen that 3D printing is becoming more and more commonplace in the healthcare sector. From surgical guides to prostheses and more, additive manufacturing’s flexibility and ability to customize are highly valued in the industry. That being said, it has not been adopted everywhere, not the least because standardization for medical 3D printing have not been fully developed. That however may soon change thanks to researchers at South Korea’s Electronics and Telecommunications Research Institute (ETRI). They have been taking the lead in developing international standards for both medical 3D printing and 3D scanning, in a move that they hope will pave the way to creating customized medical devices for each patient.
For those who are not aware, ETRI is a government-funded research institution located in Daejeon, Korea. According to its website, it is dedicated to contributing to South Korea’s economic and social development through research, distribution and development in industrial core technologies. In this capacity, they have also been looking into the standardization of 3D printing and scanning, including in the medical field for a number of years. Since 2015, it has has been invovled in the establishment of a committee to lead international standardization. Similarly, it has been developing standards for surgical 3D printing modeling and artificial intelligence-based automation since 2019. This most recent announcement of the approval of three new international standard development tasks as well as the soon-to-be finalization for other international standards shows the intent to strength Korea’s leadership in this sector.
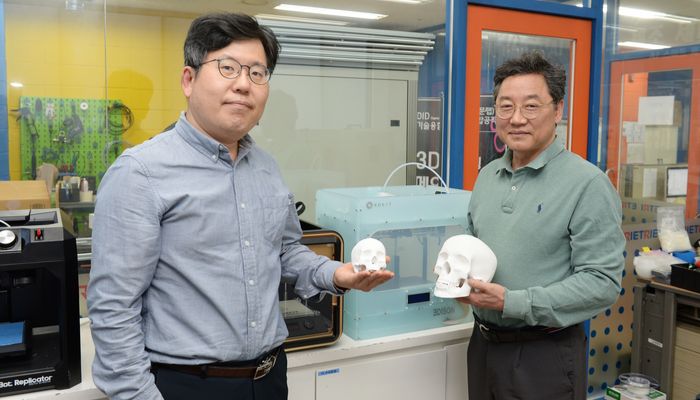
The ETRI research team shows the result of medical 3D printing (photo credits: ETRI)
The Importance of Medical Standardization
Though it can be more controversial than standarization in other fields, as it is seen as possibly preventing more innovation, medical standards are still critically important. Through this process, healthcare products and services are able to be evaluated by key stakeholders to ensure patient quality. In this case, the ETRI researchers believe that creating international standardization for medical 3D printing and 3D scanning could be of great help in promoting public health as well as revitalizing the medical equipment industry.
According to the press release, there have been three nearly adopted standardized items by ETRI: a standard evaluation process for precision/accuracy evaluation in the manufacturing process of medical 3D printing implants based on standard CT images; a precision/accuracy error evaluation method in the human tissue segmentation stage and 3D modeling stage; and a standard operating procedure for creating a data set. This means that rather than need to prepare printing models by hand, currently necessary as it can be difficult to distinguish tissue parts in the image, they would instead by able to turn to standardized procedures and methods for medical 3D printing modeling software. This would reduce design time from 24 hours to 3 while also ensuring comprehensive quality control. As experts from the FDA (US), RSNA and DICOM are also expected to participate in this standardization work, the effects could be felt internationally.
Hyung-Jun Kim, the head of ETRI’s Intelligent Convergence Research Institute, concluded: “It is very meaningful to develop more than 5 medical 3D printings essential for patient-tailored medical care led by Korea, establish a 3D scanning group, and lead the core international standards that can lead to digital twins and metaverse.”
*Cover Photo Credits: ETRI



